INTRODUCTION TO DOLICHOL-BASED GLYCOSYLATION
The covalent attachment of sugar units to proteins is a complex posttranslational modification found in all domains of life. N-glycosylation, C-mannosylation and O-mannosylation are prevalent and highly conserved glycosylation pathways, all essential for vertebrate development. The three pathways start in the endoplasmic reticulum, are based on the lipid dolichol and compete for both mannosyl donor substrates and acceptor proteins, which in many cases receive more than one type of glycan. Along these three glycosylation routes, today we know of endoplasmic reticulum associated defects in over 30 genes that cause devastating multisystemic disease manifestations (congenital disorders of glycosylation (CDG-I)), underlining the importance of these processes.
So far, the three mentioned glycosylation pathways have been studied independently from each other. However, to understand the complexity of glycosylation disorders, it is imperative to decipher the details of the dolichol-based N-glycosylation, C- and O-mannosylation pathways, as well as the precise conditions for their interconnections. To address these questions on the molecular, cellular and organismal level, we have not only brought together experts in N-glycosylation, C- and O-mannosylation, but also in structural and lipid biochemistry, in proteomics, glycoproteomics, glycomics and lipidomics, as well as in developmental biology. In the frame of the collaborative Research Unit, we can thus make use of the newest methodological developments in the different fields. Our vision is to gain a deeper understanding of dolichol-based glycosylation to facilitate the development of diagnostic tools and new therapeutic approaches.
Dolichol-based protein glycosylation
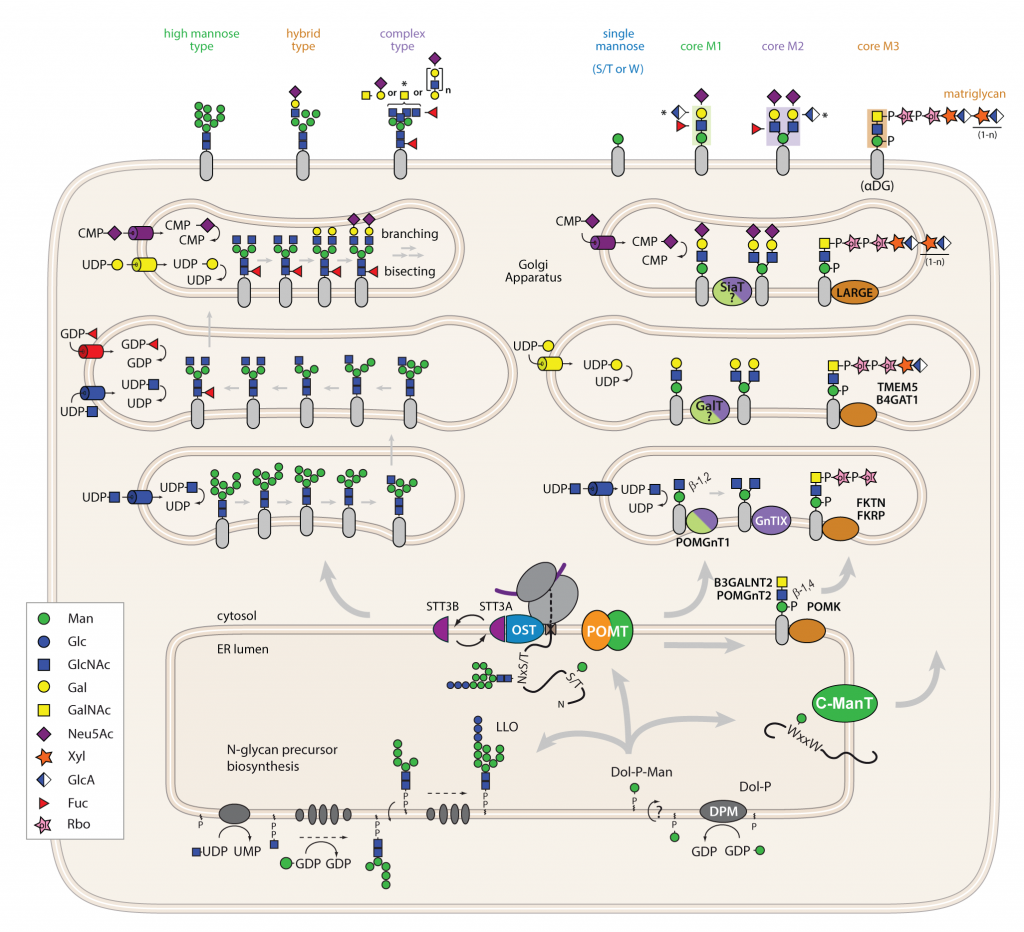
Dolichol serves as a carrier for Dol-P activated saccharides which are synthesized with UDP-GlcNAc, GDP-Man and UDP-Glc. Phosphomannomutase 2 (PMM2) catalyses the conversion of Man‑6-P into Man-1-P, the precursor molecule for GDP-Man. GDP-Man is assembled into Dol-P-Man at the cytosolic face of the ER membrane by Dol-P-Man synthase (DPM) and then translocated across the membrane bilayer. In mammals Dol-P-Man utilization is supported by protein mannose- phosphate dolichol utilization 1 (MPDU1). GDP-Man and Dol-P-Man are key substrates in N-glycosylation, O-mannosylation and C- mannosylation, each of which is essential for mammalian development and growth (reviewed in Moremen et al., 2012 and Neubert and Strahl, 2016). N-glycosylation starts with the assembly of the N-glycan precursor Glc3Man9GlcNAc2 on Dol-P (referred to as lipid-linked oligosaccharide, LLO). This process involves nucleotide- and Dol-P-activated monosaccharides as well as several highly specific glycosyltransferases (ALGs (asparagine-linked glycosylation)) that face both sides of the ER membrane (see also Appendix A1). In the ER lumen, the oligosaccharide is transferred en bloc by the oligosaccharyltransferase (OST) complex to an asparagine within the consensus sequence N-x-S/T of an acceptor protein. Protein synthesis, transport, and N-Glyco are coupled by the formation of a complex consisting of a ribosome, the Sec61 protein-conducting channel, and OST. O-mannosylation is initiated by the PMT-family of protein O-mannosyltransferases that transfer mannose from Dol-P-Man to Ser and Thr residues on nascent polypeptide chains. The classic PMT-family is evolutionarily conserved among bacteria, fungi, animals and humans. Very recently it became evident that metazoans have two other additional distinct O-Man pathways: an ER-located pathway directed by the TMTC1-4 genes that is primarily dedicated to the cadherin superfamily; and a yet uncharacterized pathway specific for plexins. C- mannosylation is unique in that a C-C bond is formed between a mannose and the indole C2 carbon of the first Trp residue in the W-x-x-W/C consensus motif. The C-mannosyltransferases which use Dol-P-Man as the donor substrate are conserved in metazoans with four representatives in mammals (DPY19L1-4). Glycoproteins can only leave the ER and travel through the Golgi apparatus to their final destinations if they are glycosylated and folded (two processes that are intricately linked). N-linked and PMT-based O-mannosyl glycans can be trimmed and/or elongated, while C-linked mannoses are not further modified. In this way, cell-, tissue- or organ-specific glycoproteomes of immense variety and information content are created.