Involvement of C-Mannosylation in Protein Processing in the Endoplasmic Reticulum and Implications for Specific Target Proteins
C-mannosylation of tryptophan (W) residues of proteins is an animal-specific glycosylation process that occurs in the endoplasmic reticulum (ER). It uses dolichol-phosphate-mannose as sugar donor, which is also required for N-glycosylation and O-mannosylation. Virtually nothing is known about the C-mannosylation process in the ER and the function of C-mannosylation of individual proteins. Only recently, we identified the gene encoding the C-mannosyltransferase. As it is related to the enzyme responsible for N-glycosylation, mechanistic and functional resemblances might exist between the two processes. C-mannose is found on a specific target sequence (WxxW), often occurring as a double motif (WxxWxxW) in which all tryptophans can be C-mannosylated.
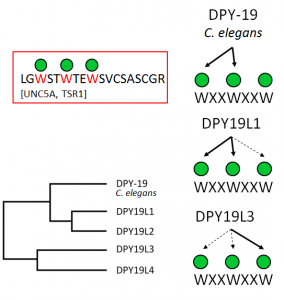
Most invertebrates have one C-mannosyltransferase gene, named dpy-19 after the dumpy phenotype of the C. elegans mutant, but mammals have four homologous enzymes. We have shown that mammals require two enzymes, DPY19L1 and DPY19L3, to fully mannosylate all three tryptophans of the WxxWxxW motif. DPY19L1 uses the first two tryptophans and DPY19L3 is responsible for the modification of the third tryptophan (Figure). In FOR 2509, we will investigate the C-mannosylation process in the ER. Is it happening co- or post-translationally, are there cofactors involved, and how is it related to N-glycosylation and O-mannosylation? We have indications that C-mannosylation aids the folding and stability of proteins, which provides an explanation for the temperature-dependent dpy-19 phenotype in C. elegans . We will further study the function of C-mannosylation for individual proteins after biosynthesis by biophysical methods and functional assays and prove whethermultiple C-mannosesprovide a recognition motif that is similar to mannoses of N-glycans or O-mannoses.
Team
Hans Bakker (Pi) is an expert on glycosyltransferases. During his PhD in Amsterdam, he worked on the galactosyltransferase family and over the last years, he has identified several new glycosyltransferases including the xylosyl-transferases responsible for glycosylation of Notch EGF repeats. Recently, he identified the first enzyme responsible for C-mannosylation, which is our subject in FOR2509.
Aleksandra Shcherbakova worked as a PhD student in our group and now started to work as a postdoc on C-mannosylation in FOR2509.
Jan Hütte studied biochemistry in Hannover and now works on C-mannosylation in our group to obtain his PhD.
Birgit Tiemann is a technician with a great experience in cloning and protein purification who supports us in the project.
For more information on Hans Bakker’s lab go to his website.
Collaborations
In vitro assays will be set up with P8 Strahl and Co-IP experiments with P9 Thiel and P8 Strahl. Isolated proteins will be analysed by MS by P5 Ruppert. Effects of C-mannosylation will be studied by us in cell culture systems, by P10 Wittbrodt in a fish model and by P3 Büttner in stem cells and experiences will be exchanged. NMR of C-mannosylated proteins is carried out with P6 Schwalbe and MS with P3 Büttner and P4 Rapp. C-Mannose negative cells will be provided P2 Brügger for lipidomics and P4 Rapp for glycomics. We have a shared interest with P7 Sinning that will structurally analyze O-mannosyltransferases, as C-mannosyltransferases have significantly conserved features.

