Characterization of induced pluripotent stem cell-based disease models for congenital disorders of glycosylation
Three ER protein glycosylation pathways (N-glycosylation, C-mannosylation, and O-mannosylation) use the donor sugar dolicholphosphate-mannose (Dol-P-Man). Dol-P-Man is synthesized from mannose via Man-6-P, Man-1-P and GDP-Man. Isomerization of Man-6-P to Man-1-P is catalyzed by phosphomannomutase 2 (PMM2). Hypomorphic mutations in PMM2 cause the most frequent form of congenital disorder of glycosylation, PMM2-CDG, a severe multisystemic disorder affecting with high penetrance nervous system and liver. So far defects in N-glycosylation are regarded as causative for PMM2-CDG. Considering, however, that the three pathways merge in their need for Dol-P-Man and also C-mannosylation and O-mannosylation are known to impact folding and functionality of proteins with major roles in development (e.g. cadherins and cytokine receptors are O-mannosylated and C-mannosylated, respectively) it is likely that the PMM2-CDG syndrome combines alterations in all these pathways. We recently established induced pluripotent stem cells from PMM2-CDG fibroblasts (PMM2-iPSCs) and demonstrated alterations in N-glycosylation. Here we suggest using these cells together with controls to comparatively investigate the three Dol-P-Man dependent pathways by applying established as well as specifically adapted protocols in glycoproteomics. With a focus at the organs chiefly affected in PMM2-CDG patients, nervous system and liver, we will perform time laps analyses starting from human pluripotent stem cells (hPSCs) and continuing over different stages during neural and hepatic differentiation.
To identify target proteins of C-mannosyltransferases and analyse its functional role in early steps of human development, we plan to establish C-mannosyltransferase-deficient hPSCs. Using both, the available PMM2-CDG and C-mannosyltransferase-deficient stem cell models, we will finally address the question if alterations in these pathways reflect at transcriptomic level. The rationale behind this approach bases on the fact that we expect that aberrant glycosylation of cytokines or membrane receptors causes perturbations on gene expression that can be deciphered by bioinformatics analyses of quantitative gene expression data.
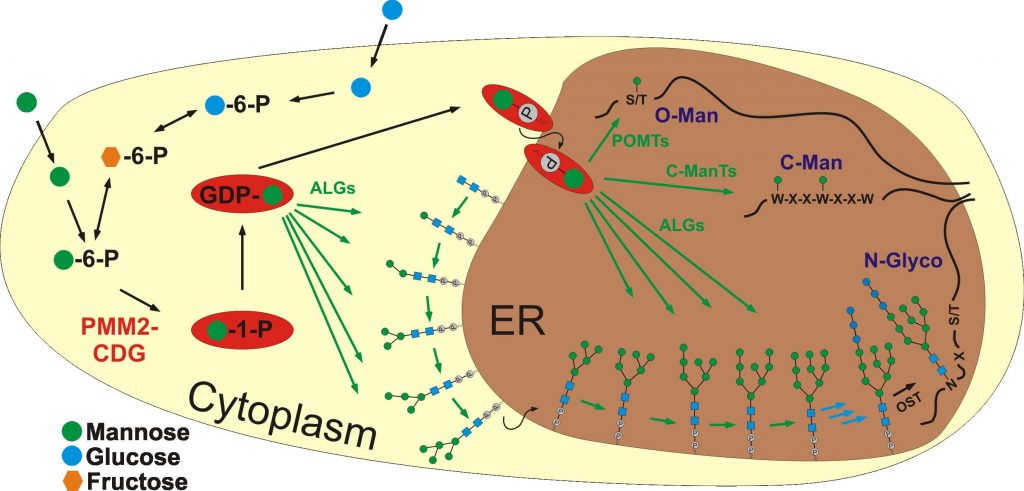
Biosynthesis of N-linked, C- and O-mannosyl glycans in mammals. Mutations in the enzyme PMM2 causing PMM2-CDG lead to reduced levels of the mannosyl donors GDP-Man and Dol-P-Man (highlighted by red ovals) thereby affecting all three types of glycosylation (N-Glyco, C-Man and O-Man).
Team

Falk Büttner (Pi) is a glycobiologist with a strong background in analytics who recently developed a novel approach for the analysis of glycosphingolipid glycosylation. He has contributed to the identification and characterization of several glycosyltransferases including the first C-mannosyltransferase, DPY-19 from C. elegans.
Karsten Cirksena studied Biology and Biomedicine in Hamburg and Hannover. He specialized on the application of CRISPR-Cas for genome editing in human pluripotent stem cells. He is also performing (glyco)proteomic analyses.
Charlotte Rossdam is a biochemist and has earned her PhD in Hannover. She is working with PMM2-CDG iPSCs and became expert in hepatic and cardiomyogenic differentiation of human pluripotent stem cells.
For more information on Falk Büttner’s group visit his website.
Collaborations
- Expression of target proteins for C-mannosylation in mutant hPSCs will be done together with Hans Bakker (P1)
- We will collaborate with Sabine Strahl (P8) to analyze the influence of PMM2-CDG on O-mannosylation.
- N-glycosylation site occupancy at a whole cell level will be analyzed in collaboration with Erdmann Rapp (P4).
- Isolated glycopeptides will be analyzed by Thomas Ruppert (P5).
- Samples of PMM2-CDG iPSCs and control iPSCs at different stages during neuronal differentiation will be given to Britta Brügger (P2) for analysis of lipids.
- N-glycosylation in C-mannosyltransferase-deficient hPSCs will be analyzed by Erdmann Rapp (P6)
