Protein O-Mannosylation and its diverse interplay with N-Glycosylation
Protein O-mannosylation and N-glycosylation are fundamental processes that are essential for the growth and development of eukaryotes. Both protein modifications initiate at the endoplasmic reticulum. Properly glycosylated and folded proteins can journey on to the Golgi apparatus where N-linked and O-mannosyl glycans are further trimmed and/or elongated. Ultimately, glycoproteins with highly diverse glycans emerge. The importance of these protein modifications becomes obvious in light of the fact that defects in both types of glycosylation result in severe human diseases such as the congenital disorders of glycosylation (CDGs).
In the endoplasmic reticulum, the N-glycosylation machinery and protein O-mannosyltransferases compete for the sugar donor dolichol phosphate-activated mannose. There is now growing evidence that the connection between O-mannosylation and N-glycosylation extends far beyond their sharing of a common sugar donor (reviewed in Neubert and Strahl, 2016). Our recent work revealed that the inhibition of mammalian protein O-mannosyltransferases negatively affects E-cadherin-mediated cell-cell adhesion, via unexpected crosstalk between O-mannosylation and the biosynthesis of individual N-glycan structures on this important adhesion molecule (Lommel et al., 2013; Carvalho et al., 2016). It is still unclear, however, whether the observed effect is restricted to E-cadherin or whether N-glycosylation is generally affected by a decrease in O-mannosylation, and what mechanisms are behind this coordinated interplay.
To address these questions, we aim to study mammalian O-mannosylation and its relation to N-glycosylation at the operating, regulatory and functional levels.
Our main objectives are
- unravel the mode of action of the mammalian O-mannosyltransferases in the endoplasmic reticulum
- explain the consequences of defective O-mannosylation at the molecular level with a focus cell adhesion moelcules
In combination, our analyses will provide a comprehensive view of the initiation of O-mannosylation in the endoplasmic reticulum as well as the diverse implications of O-mannosylation defects, and guide the analyses of physio-pathologic abnormalities in CDG models.
Neubert & Strahl (2016). Curr Opin Cell Biol. 41:100-8; Lommel et al. (2013). Proc Natl Acad Sci U S A. 110:21024-9; Carvalho et al. (2016) Oncotarget. 7:65231-65246.
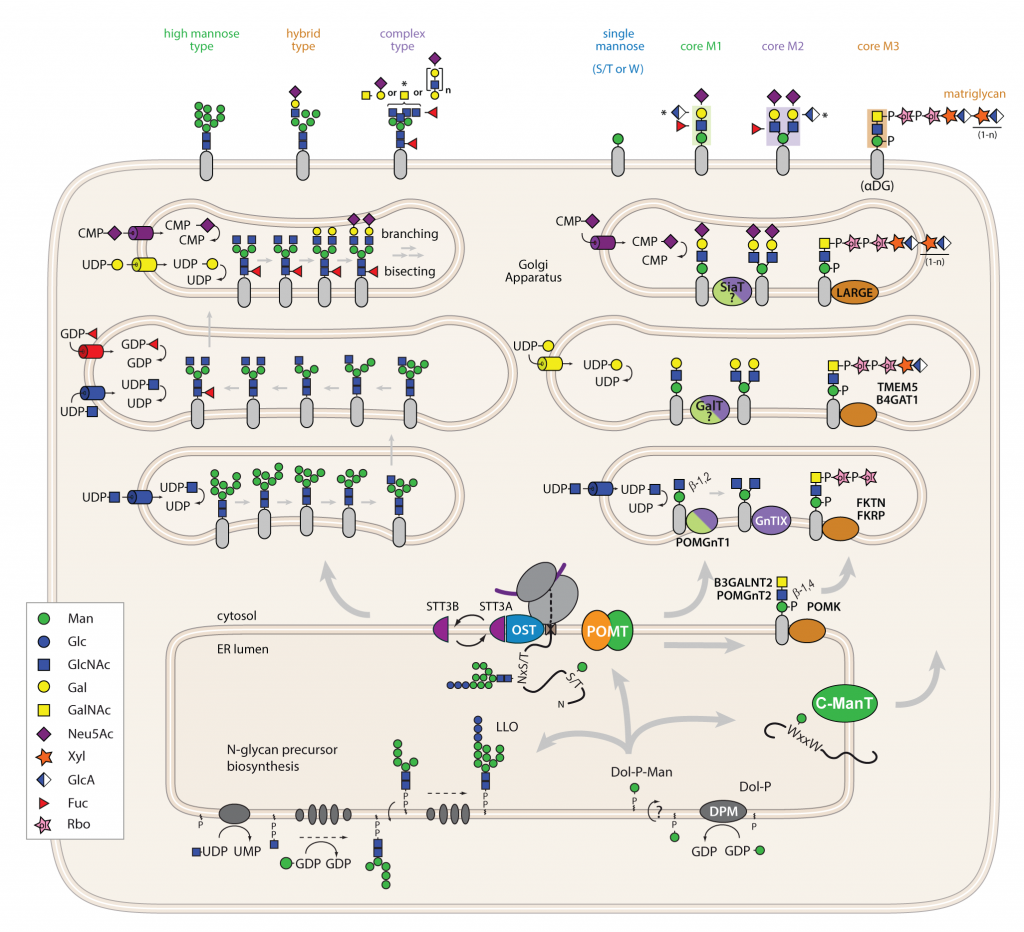
Figure 1: The biosynthetic pathway of N-linked and O-mannosyl glycans in mammals.
In the ER, assembly of the mannose donor Dol-P-Man and the N-glycan precursor Glc3Man9GlcNAc2-PP-dolichol take place. At the translocon complex, the oligosaccharide is transferred by the oligosaccharyltransferase complex (OST) to Asn present in the consensus sequence N-x-S/T of acceptor proteins. Further trimming steps of the N-glycan in the ER are not illustrated. O-Man is initiated by POMT complexes by the transfer of mannose from Dol-P-Man to Ser and Thr residues of acceptor proteins. Whether mammalian POMTs also act at the translocon is not known. In mammals, upon ER exit, the initial protein-linked glycans may be extended step-wise. Numerous different glycosyltransferases contribute to this process. During N-Glyco high mannose, hybrid and complex type glycans and their derivatives are assembled (left panel). During O-Man core M1, M2 and M3 glycans, and their derivatives are synthesized. In the absence of the β1,2-GlcNAc transferase POMGnT1 no extended core O-mannosyl glycans are formed (right panel). (gift from P. Neubert)
Team

Sabine Strahl (PI) is a glycobiologist who´s laboratory has substantially contributed to the basic understanding of protein O-Man in yeast and mammals.
Shahidul Alam (PostDoc) has earned his PhD at University Hospital Basel. He is involved in structure – function analyses of PMT and POMT complexes.
Lina Siukstaite (PostDoc) is exploring the correlation between glycosylation and glycolysis processes. She earned her PhD as a Marie Curie fellow at the University of Freiburg in Germany.
Daniel Sturm (PhD student) is studying the interplay of O-mannosylation and N-glycosylation.
For more information on Sabine Strahl’s lab go to his website.
Collaboration
We are planning to perform proteomics, glycoproteomics and lipidomics analysis in collaboration with the groups of Thomas Ruppert (P5), Erdmann Rapp (P4) and Britta Brügger (P2), respectively. ALG-CDG patient cells, glycosylation deficient hiPSC and fish models will be studied in collaboration with the groups of Christian Thiel (P9), Falk Büttner (P3) and Jochen Wittbrodt (P10), respectively. Our tools will support structural studies of the groups of Irmi Sinning (P7) and Harald Schwalbe (P6).
