Unravelling the correlation between different ER-based types of glycosylation via glyco/proteo-analytics
Within the FOR2509 our aim is to provide state-of-the-art analytical techniques to investigate the impact of mutations in genes for glycosyltransferases and glycosidases on the ER-based glycosylation network for N‑glycosylation, C- and O-mannosylation. Mutations in these enzymes lead to a variety of disease called congenital disorder of glycosylation (CDG). Our group has specialized on the field of glyco/proteo-analytics where we are applying a comprehensive set of high performance tools for proteomics (2D-DIGE, MALDI-TOF/TOF-MS/MS, RP-LC-MS/MS), glycoproteomics (RP-LC-MSn) and glycomics (xCGE-LIF, HILIC-FLR, CE-MS, PGC-LC-MS/MS, MALDI-TOF/TOF-MS/MS, RP-LC-MS/MS). This toolbox, consisting of orthogonal (electrophoresis-, chromatography- and/or mass spectrometry-based) methods and dedicated software-tools developed for this purpose, will enable us to study all types of glycosylation in the context of this project (Figure 1).
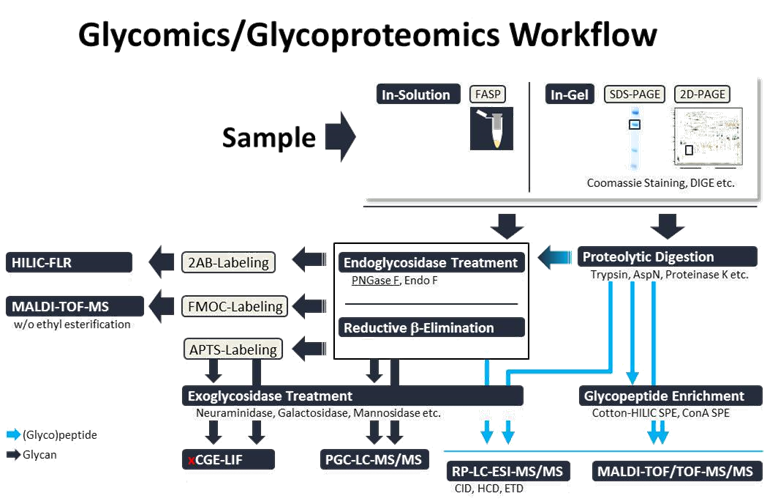
Figure 1: Glycomics/Glycoproteomics Workflow.
For N-glycomics we are employing multiplexed capillary gel electrophoresis with laser-induced fluorescence detection (xCGE-LIF), which has become increasingly popular as a standalone method, most likely due to its HTP capabilities.
In addition to xCGE-LIF we employ orthogonal methods, like matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and hydrophilic interaction chromatography with fluorescence detection (HILIC-FLR) for the qualitative and quantitative analysis of released and fluorescently-labelled N-glycans. O-glycans are analyzed by a liquid chromatography tandem MS (LC-MS/MS) workflow that combines the isomeric separation of non-labelled glycans via porous graphitized carbon liquid chromatography (PGC-LC) with the structural information that can be gained from subsequent high-resolution multistage mass spectrometry (MSn). For determination of all occupied glycosylation sites (macroheterogeneity) of a given protein, as well as the characterization and quantification of the individual glycan structures (microheterogeneity) we are using a reversed phase (RP) LC-MSn method with CID (low-energy collision-induced dissociation) and ETD (electron-transfer dissociation) fragmentation.
By applying these tools onto the samples generated and supplied by our cooperation partners, we are aiming for a deeper understanding of the interactions and the hierarchy of the different glycosylation pathways.
Team



Erdmann Rapp (Pi) studied chemistry at the universities of Konstanz and Tübingen. During his PhD, he was stipendiary of DFG graduate school for analytical chemistry and specialized on miniaturized separation techniques coupled to MS and NMR. He was invited research fellow at the NMR Centre of Wageningen University (The Netherlands), before he got research associate and head of the Laboratory for Miniaturized Separation Techniques at the Institute of Process Engineering of the Otto von Guericke University (OvGU) in Magdeburg. Since 2003, he is head of Bio/Process Analytics at Max Planck Institute for Dynamics of Complex Technical Systems (MPI-DKTS) in Magdeburg. He and his team are working on the development and implementation of innovative cutting-edge bio/process analytical tools for a deeper understanding of bio(techno)logical processes – in particular, on sophisticated tools for proteomics, glycoproteomics and glycomics to be able to handle the highly complex samples arising along bioprocess development, biomedical research and biomarker discovery.
Marcus Hoffmann has received his Diploma at the OvGU, where he studied in Biosystems Engineering. During his PhD he specialized on mass spectrometry-based N- and O-glycoproteomics. During the DFG-project he will be involved in the application of those techniques for the in-depth glycoproteomic analysis of CDG-related glycoproteins.
Valerian Grote has received the Bachelor of Science from the University of Applied Science Coburg, where he has studied Bioanalytics with a focus on mass spectrometry-based proteomics. He has finished a graduate program in human- and molecular biology at the Saarland University with a master thesis about the analysis of N‑glycans at the MPI-DKTS, where he is currently enrolled in a PhD program.
For more information on Erdmann Rapp’s lab visit his website.
Collaborations
We are going to exchange samples with the research groups of Hans Bakker (P1), Falk Büttner (P3), Sabine Strahl (P8), Christian Thiel (P9) and Joachim Wittbrodt (P10) to look at the global N-glycome of model systems deficient for N-glycosylation, C- or O-mannosylation.
To investigate the mutual influence of N-glycosylation, C- and O-mannosylation in model proteins, we are going to work closely with the research groups of Hans Bakker (P1), Falk Büttner (P3), Thomas Ruppert (P5), Sabine Strahl (P8) and Christian Thiel (P9).
For the analysis of N-glycosylation, C- and O-mannosylation in model systems with reduced levels of mannose donors, we are going to collaborate with the research groups of Hans Bakker (P1), Falk Büttner (P3), Thomas Ruppert (P5), Christian Thiel (P9) and Joachim Wittbrodt (P10).
